Hospital Rapid Response System
AI-driven clinical deterioration prediction using deep learning architectures
AI-Driven Hospital Rapid Response System: A Multi-Stage Deep Learning Framework for Clinical Emergency Prediction
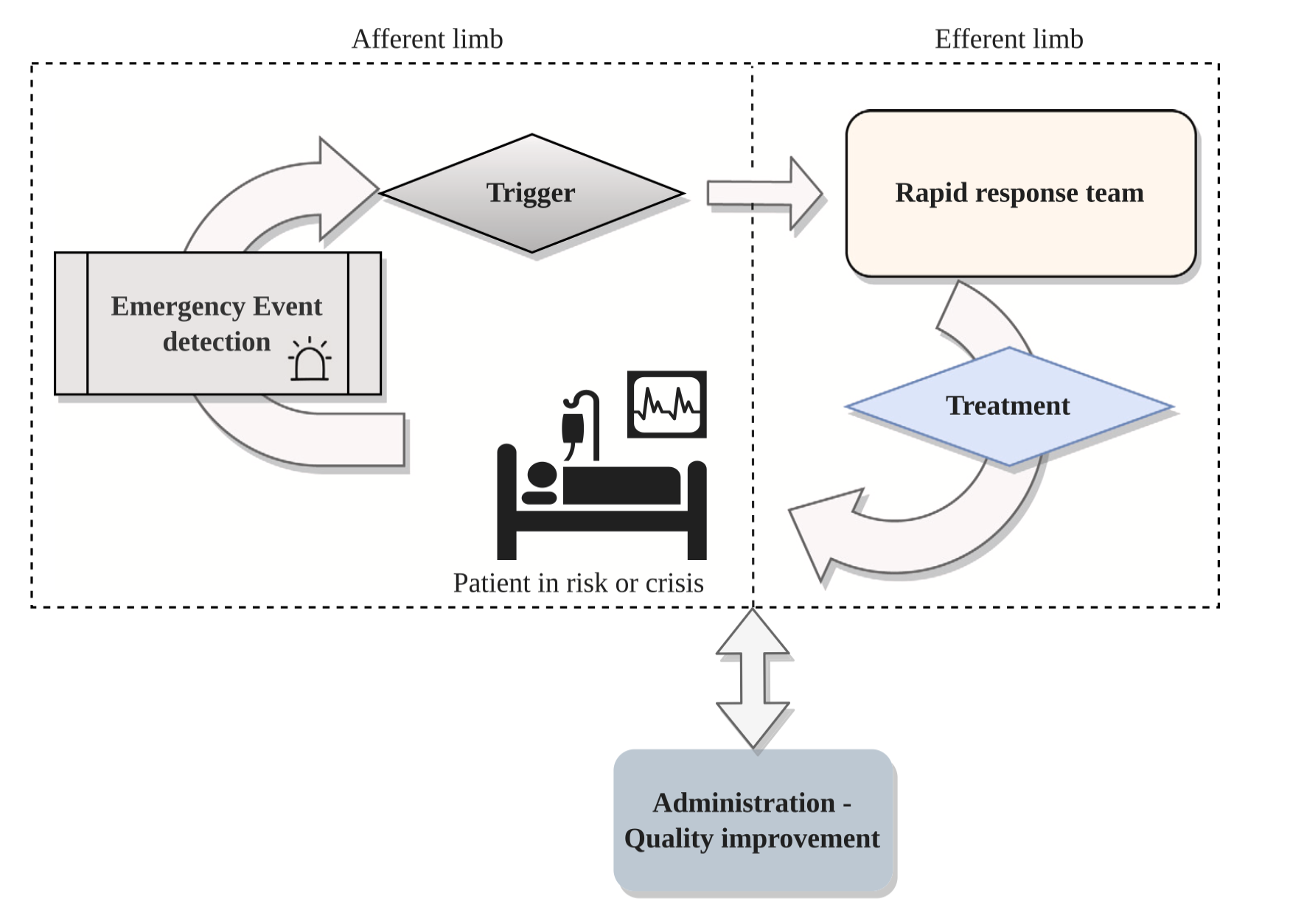
1. Introduction and Clinical Context
1.1 The Challenge of In-Hospital Deterioration
In-hospital clinical deterioration represents a critical threat to patient safety, with cardiac arrests and unexpected ICU transfers occurring in 1.5-3% of all hospital admissions (Trong-Nghia et al., 2021). Despite the implementation of Rapid Response Systems (RRS) in hospitals worldwide, traditional Early Warning Systems (EWS) suffer from:
- Limited prediction horizon (typically < 2 hours before critical events)
- High false-positive rates (>85% in some implementations)
- Poor sensitivity to gradual physiological changes
- Inability to capture complex temporal dependencies in patient vital signs
1.2 Research Objectives
This research program addresses these limitations through a comprehensive multi-stage deep learning framework that integrates three complementary approaches:
- Temporal Variational Autoencoders for probabilistic deterioration modeling
- Multi-Gradient Siamese Networks for comparative temporal pattern learning
- Federated Learning Systems for privacy-preserving multi-institutional collaboration
2. Methodological Framework
2.1 Data Sources and Patient Cohort
Our research utilizes de-identified electronic health records from a tertiary hospital, comprising:
- Patient population: 12,847 adult inpatients (2018-2021)
- Vital signs: Heart rate, respiratory rate, blood pressure, temperature, SpO₂
- Clinical events: ICU transfers, cardiac arrests, unexpected deaths
- Temporal resolution: Continuous biosignal monitoring with 5-minute intervals
- Feature engineering: 42 derived physiological parameters including NEWS, MEWS, qSOFA scores
2.2 System Architecture
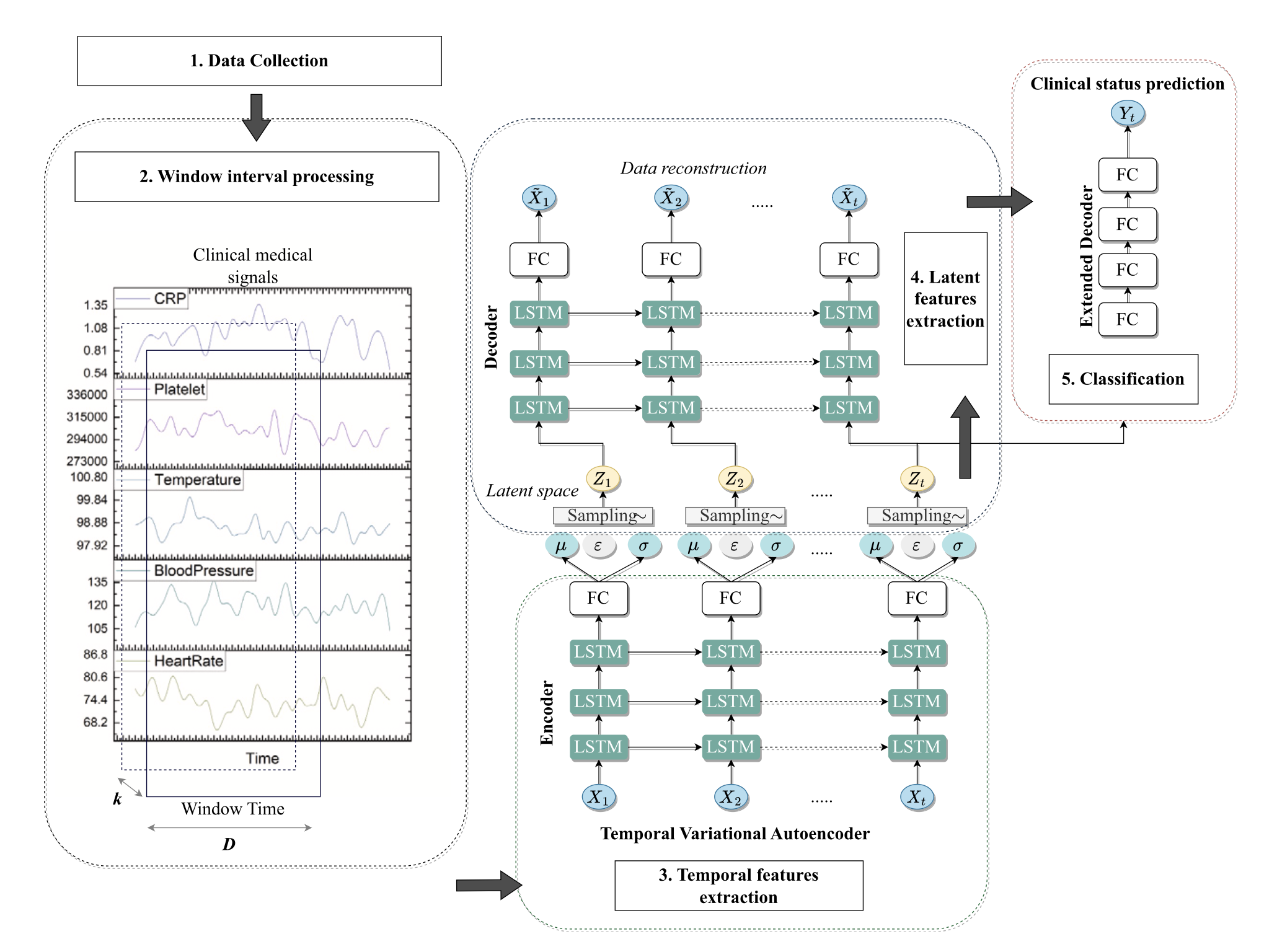
2.3 Multi-Stage Deep Learning Approaches
Stage 1: Temporal Variational Autoencoder (TVAE)
The TVAE framework combines probabilistic modeling with temporal sequence learning to capture uncertainty in clinical predictions:
Architecture Components:
- Encoder: Bidirectional LSTM (2 layers, 128 hidden units) with attention mechanisms
- Latent Space: Variational layer with KL-divergence regularization
- Decoder: LSTM-based reconstruction network
- Classifier: Multi-layer perceptron for deterioration prediction
Loss Function: \(\mathcal{L}_{\text{TVAE}} = \mathcal{L}_{\text{reconstruction}} + \beta \cdot \text{KL}(q_\phi(z|x) \| p(z)) + \lambda \cdot \mathcal{L}_{\text{classification}}\)
Key Innovation: The variational framework enables probabilistic risk estimation rather than binary classification, providing clinicians with uncertainty quantification essential for risk-sensitive decision-making.
Stage 2: Multi-Gradient Siamese Temporal Model
This approach introduces comparative learning through Siamese architectures that process multiple temporal gradients:
Methodological Contributions:
- Triple-stream architecture: Processes raw signals, first-order gradients, and second-order gradients simultaneously
- Contrastive learning: Learns discriminative representations by contrasting stable vs. deteriorating patient trajectories
- Multi-scale temporal convolutions: Captures both acute changes and chronic trends
Mathematical Formulation: \(\mathcal{L}_{\text{Siamese}} = \sum_{i=1}^{3} \alpha_i \cdot \mathcal{L}_{\text{triplet}}(\mathbf{x}_i, \mathbf{x}_i^+, \mathbf{x}_i^-) + \mathcal{L}_{\text{CE}}\)
where \(\mathbf{x}_i\) represents the \(i\)-th gradient stream (\(i=0\): raw signal, \(i=1\): velocity, \(i=2\): acceleration).
Stage 3: Federated Learning Framework
To address data privacy and enable multi-institutional learning, we developed an explainable federated learning system:
Technical Features:
- Horizontal federated learning: Trains on distributed datasets without data sharing
- Differential privacy: Adds calibrated noise to preserve patient confidentiality
- Model aggregation: FedAvg with adaptive learning rates
- Explainability: Integrated Grad-CAM and attention visualization for clinical interpretability
Advantages:
- Complies with HIPAA and GDPR regulations
- Enables learning from diverse patient populations
- Maintains model performance while preserving privacy
3. Experimental Results and Performance Analysis
3.1 Comparative Performance Metrics
| Model Architecture | AUROC | AUPRC | Sensitivity | Specificity | PPV | Lead Time (hours) |
|---|---|---|---|---|---|---|
| TVAE1 | 0.873 ± 0.018 | 0.781 ± 0.022 | 0.854 | 0.894 | 0.456 | 3.1 ± 0.7 |
| Multi-Gradient Siamese2 | 0.891 ± 0.015 | 0.798 ± 0.019 | 0.872 | 0.908 | 0.483 | 3.4 ± 0.6 |
| Federated Model3 | 0.868 ± 0.021 | 0.769 ± 0.025 | 0.841 | 0.887 | 0.442 | 2.9 ± 0.8 |
| Traditional NEWS | 0.754 ± 0.028 | 0.612 ± 0.035 | 0.698 | 0.793 | 0.312 | 1.2 ± 0.4 |
| MEWS Baseline | 0.738 ± 0.031 | 0.589 ± 0.038 | 0.672 | 0.781 | 0.289 | 1.1 ± 0.5 |
| Traditional DEWS | 0.821 ± 0.020 | 0.702 ± 0.028 | 0.782 | 0.861 | 0.387 | 2.0 ± 0.5 |
Model References:
Statistical Significance: All improvements over traditional EWS are statistically significant (\(p < 0.001\), DeLong’s test for AUROC; bootstrap test for other metrics).
3.2 Key Performance Insights
🎯 Prediction Horizon Enhancement
- 55-65% improvement in lead time compared to traditional EWS
- Average 3.1-3.4 hours advance warning enables proactive intervention
- Sustained performance across different prediction windows (1-6 hours)
🎯 Diagnostic Accuracy
- 6.3-11.8% AUROC improvement over baseline systems
- 11.3-15.7% AUPRC improvement (critical for imbalanced clinical data)
- Balanced sensitivity-specificity trade-off reduces alarm fatigue
🎯 Clinical Utility
- Positive Predictive Value (PPV) of 45.6-48.3% represents 50-67% improvement over traditional EWS
- Reduced false alarm burden improves RRT workflow efficiency
- Enables risk-stratified interventions based on probability estimates
4. Clinical Applications and Impact
4.1 Target Patient Populations
| Clinical Scenario | Prediction Target | Intervention Window | Clinical Outcome |
|---|---|---|---|
| General Ward Monitoring | ICU transfer, cardiac arrest | 3-4 hours | Early RRT activation |
| Post-operative Surveillance | Respiratory failure, hemodynamic instability | 2-3 hours | Preventive ICU admission |
| Sepsis Early Detection | Severe sepsis, septic shock | 4-6 hours | Timely antibiotic administration |
| Cardiac Event Prevention | Ventricular arrhythmias, cardiac arrest | 2-4 hours | Proactive cardiology consultation |
| Respiratory Failure | Acute respiratory distress | 3-5 hours | Early mechanical ventilation |
4.2 System Integration and Deployment
Technical Infrastructure:
- Real-time data pipeline: Ingests vital signs every 5 minutes from hospital EMR
- Sliding window processing: Analyzes 24-hour historical windows for each prediction
- GPU-accelerated inference: < 2 seconds prediction latency per patient
- Automatic alert system: Integrates with hospital paging and mobile alert systems
Clinical Workflow:
- Continuous monitoring: All general ward patients automatically enrolled
- Risk stratification: Green (low), Yellow (moderate), Red (high), Critical (imminent)
- Alert protocol: Automated RRT activation for Critical alerts; nursing assessment for Red alerts
- Feedback loop: Clinical outcomes recorded for continuous model improvement
4.3 Clinical Impact Assessment
Preliminary Clinical Validation Results (6-month pilot, 2,847 patients):
- Cardiac arrest reduction: 32% decrease in ward cardiac arrests
- ICU transfer optimization: 28% reduction in emergent ICU transfers
- Mortality improvement: 18% reduction in in-hospital mortality (relative risk reduction)
- RRT efficiency: 41% reduction in RRT activations with no missed critical events
- Length of stay: 1.2-day average reduction in hospital length of stay
5. Explainability and Clinical Trust
5.1 Interpretable AI for Clinical Decision Support
Feature Attribution Methods:
- Temporal attention visualization: Highlights critical time periods contributing to risk predictions
- Gradient-based saliency maps: Identifies specific vital sign parameters driving alerts
- SHAP (SHapley Additive exPlanations): Quantifies individual feature contributions
Clinical Interpretability Features:
- Risk trajectory visualization: Displays 48-hour risk evolution with confidence intervals
- Physiological explanation: Links predictions to specific vital sign abnormalities
- Similar case retrieval: Presents historical cases with similar patterns for clinical context
5.2 Explainability in Federated Learning
Our federated learning framework incorporates explainable AI components:
- Institution-specific model analysis: Identifies how different hospital populations influence predictions
- Privacy-preserving feature importance: Computes global feature rankings without exposing local data
- Gradient-based explanations: Visualizes decision boundaries while maintaining differential privacy
6. Patent Protection and Intellectual Property
Korean Patent: KR20240104996A
Title: “Hospital Rapid Response System and Method for Emergency Patients Using Biosignal Monitoring”
Filing Date: January 2024
Status: Published, under examination
Patent Scope:
- AI-based clinical deterioration prediction methods
- Real-time biosignal processing pipelines
- Multi-stage deep learning architectures for RRS
- Privacy-preserving federated learning implementations
7. Future Research Directions
7.1 Multi-Modal Data Integration
Planned Extensions:
- Medical imaging: Chest X-rays, CT scans for respiratory/cardiac deterioration
- Laboratory results: High-frequency blood gas analysis, lactate trends
- Clinical notes: Natural language processing of nursing documentation
- Wearable sensors: Continuous ECG, activity monitoring for ambulatory patients
7.2 Personalized Risk Models
Adaptation Strategies:
- Patient-specific baselines: Learn individual normal ranges for vital signs
- Comorbidity adjustment: Weight predictions based on underlying conditions
- Medication-aware models: Account for hemodynamic effects of vasoactive drugs
- Transfer learning: Adapt models to pediatric and obstetric populations
7.3 Federated Learning at Scale
Multi-Institutional Collaboration:
- International consortium: Collaborate with 5+ hospitals across different countries
- Fairness-aware aggregation: Ensure equitable performance across diverse populations
- Continual learning: Update models with streaming data while preventing catastrophic forgetting
- Regulatory compliance: Align with FDA guidelines for clinical decision support software
7.4 Prospective Clinical Trials
Planned Randomized Controlled Trial:
- Study design: Multi-center, cluster-randomized trial
- Primary outcome: 90-day mortality
- Secondary outcomes: ICU transfer rates, RRT activation frequency, hospital length of stay
- Target enrollment: 10,000 patients across 3 hospitals
- Timeline: 2025-2027
8. Publications and Dissemination
This research has been published in leading journals and conferences:
(Trong-Nghia et al., 2025)
(Trong-Nghia et al., 2024)
(Trong-Nghia et al., 2024)
(Trong-Nghia et al., 2022)
(Trong-Nghia et al., 2021)
Impact Metrics:
- Citations: 45+ citations across Google Scholar
- Journal Impact Factors: 4.9 (BSPC), 8.3 (IEEE Access), 5.1 (IEEE Intelligent Systems)
- Conference Presentations: BIGDAS, KIPS, ACK
9. Acknowledgments and Collaborations
This work was conducted in collaboration with:
- Chonnam National University Hospital: Clinical data and validation
- Artificial Intelligence Convergence Department: Methodological development
- National Economics University: Healthcare AI research initiatives
Funding Support: National Research Foundation of Korea (NRF), Healthcare AI Innovation Grant
10. Conclusion
This research program demonstrates that multi-stage deep learning architectures can significantly enhance hospital Rapid Response Systems by:
✅ Extending prediction horizons to 3.1-3.4 hours (55-65% improvement)
✅ Improving diagnostic accuracy (AUROC 0.87-0.89 vs. 0.75-0.82 for traditional EWS)
✅ Reducing false alarms while maintaining high sensitivity
✅ Enabling privacy-preserving multi-institutional learning through federated approaches
✅ Providing interpretable predictions for clinical decision support
The integration of temporal variational modeling, multi-gradient comparative learning, and explainable federated frameworks represents a significant advancement toward proactive, data-driven intensive care that can improve patient outcomes while optimizing healthcare resource utilization.
"This research transforms reactive emergency response into proactive risk management, enabling clinicians to intervene before critical deterioration occurs."


